
 Combustion of fuels:
Combustion of fuels:Whwn we burn a fuel than it gains the oxygen and is oxidised.Energy use to release.For a very good example natural gas(Methane, CH4).
CH4(g)+2O2(g) gives CO2(g)+2H2O(g).
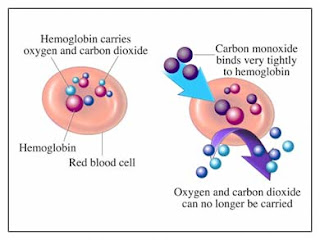
This is the ideal condition that how fuel use to burn .We cannot say that the production of the combustion is always the carbondioxide and the water.There also use to be in complete combustion which is use to happen the oxygen is not enough available.This use to happen when the boiler, fire or the engine are not properly serviced or when the fuel is blocked.This types of incomplete combustion use to be very dangerous for the human and earth and may help to increase the global warming on the earth.Incomplete combustion use to creat the carbonmonoxide instead of the the carbondioxide.The carbonmonoxide is one of the very dangerous gas.This gas is colourless and odourless.The carbonmonoxide use to combine with the haemoglobin in red blood cells, and help to stop the haemoglobin to carry the oxygen.Dizziness, headaches, sickness and tiredness or flu-like symptoms are early symptoms of carbonmonoxide poisoning.The victims use to confuse to do anything and fall asleep.The carbon monoxide poisoning is fatal and even if rescued in times after effect can be permanent.The sign of producing carbonmonoxide from the boiler is yello flame rather than the blue flame & any sign of staining, soot or burns on the outside above the burners.When anybody complain drowsiness or sickness when the fire is on then it
 must be investigated by the professional.Many people use to close all the doors and windows when the fire is on then what happen is other extra fresh oxygen cannot enter the room and those oxygen which were inside the room will finish and will not fast replace as the result their produce carbonmonoxide and human may die due to this.It is important that system are designed & installed properly.The dector of carbonmonoxide can be use to give the warning problems.You can use the following equation for the carbonmonoxide from the methane, most of the appliances used to produce the mixture of carbondioxide and carbonmonoxide.It is found that well maintained vehicles used to produce 1% carbonmonoxide in it's emission.
must be investigated by the professional.Many people use to close all the doors and windows when the fire is on then what happen is other extra fresh oxygen cannot enter the room and those oxygen which were inside the room will finish and will not fast replace as the result their produce carbonmonoxide and human may die due to this.It is important that system are designed & installed properly.The dector of carbonmonoxide can be use to give the warning problems.You can use the following equation for the carbonmonoxide from the methane, most of the appliances used to produce the mixture of carbondioxide and carbonmonoxide.It is found that well maintained vehicles used to produce 1% carbonmonoxide in it's emission.2CH4(g)+3O2(g) gives 2CO(g)+4H2O(g)
When unburnt carbon use to react with the carbondioxide, carbonmonoxide is also used to produce.
C(s)+CO2(g) gives 2CO(g)

0 comments:
Post a Comment